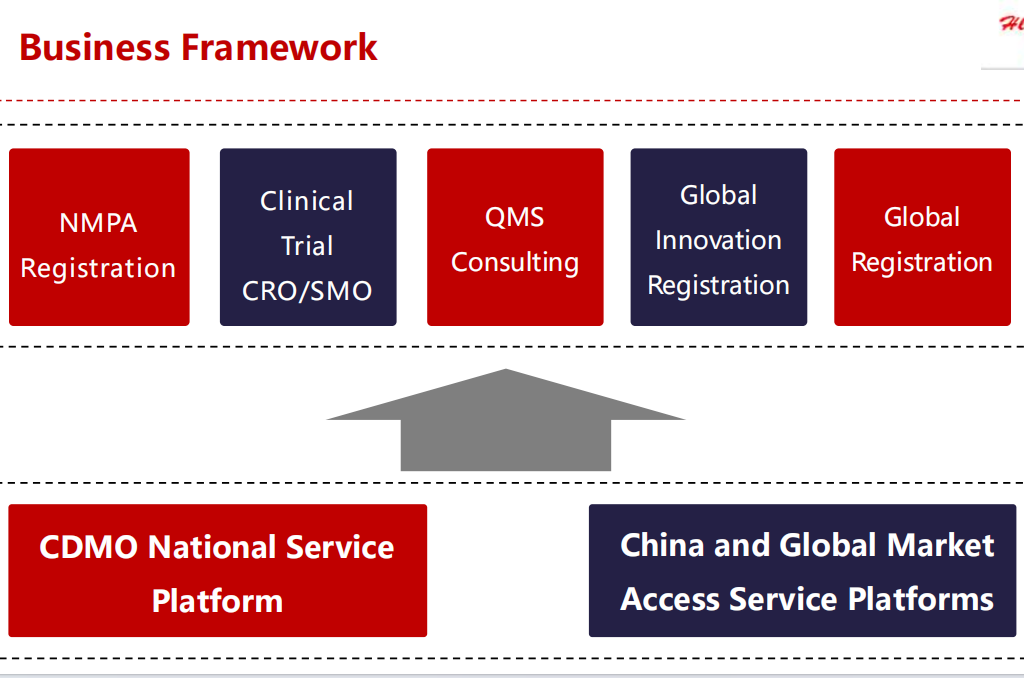
Hlongmed is a leading professional medical device regulatory partner, CDMO, clinical trial CRO/CRA/SMO/CRC cooperative organization, and a trusted professional medical device industry overall solution and CDMO service provider.
Hlongmed is headquartered in Shenzhen and has subsidiaries or offices in the United States, Ireland, Hong Kong, Vietnam, Beijing, Wuhan, Suzhou, Changsha, Chengdu, and Chongqing, as well as a professional research institute in Shenzhen. Over 3000 domestic and international medical device enterprises, Authorized parties, hospitals, NB and NGO have entrusted Hlongmed services.
With rich Experiences in Clinical trial, CDMO, regulatory compliance and procedure excellence, Hlongmed team focus on providing professional medical device services .
Hlongmed adheres to a sincere and friendly attitude and have established Long-term friendly relationship with clinical trial sites, NB, competent Authorities, Test Lab, Associations, Investor, IP and Law servicers.
Our Gallery
Event Location
Accra International Conference Centre
Contact Information
+233 (531) 035 868
Other Pages
- Home
- About Event
- Speakers
- Schedule

